(Click twice on picture to enlarge)
by John P. Pratt (1 Aug 2011)
©2011 by John P. Pratt. All rights Reserved.
This article is both an introduction to Helen Pawlowski's model of the atom and to her Circular Periodic Table, as well as a book review of her book The Visualization of the Atom (Riverton, UT: Pawlowski Family Trust, 1990). First Helen and her work are introduced, then the model's strengths and weaknesses are summarized.
In about 1990 I had the pleasure of meeting a wonderful woman named Helen Pawlowski, who lived near me in Orem, Utah. She had been working for many years on both a new Circular Periodic Table and also a way to visualize the atom. In fact, she had what was actually her own model of the atom. She asked me to read a preprint of her book The Visualization of the Atom to critique and hopefully to endorse it. She knew I have a PhD in astronomy and had written several scientific papers. She told me she had had great trouble getting anyone from academia to even give her work a second glance, much less endorse it. So I decided to look it over.
What I found was that she was an extremely intelligent woman who had not been brainwashed at our institutions of higher learning and was thus able to actually do some creative work that was literally "outside of the box". In fact, it was more "inside of a circle"! She had done some excellent research, and was familiar with the debate between Albert Einstein and Neils Bohr about whether or not the atom could even be visualized at all. Even though Bohr and the science called quantum mechanics won the debate in the eyes of the world, she was with Einstein in believing that the atom could actually be visualized. In fact, she dared to actually visualize it and write a book to show others how to do it. What resulted was a strange chart which is a hybrid of being both a Circular Periodic Table and also a way to visualize the atom at the same time!
The critique I gave of her work was that it had both merits and weaknesses, but that I didn't think the merits of her elegant table were enough to overturn the deep entrenchment of the usual Periodic Table, which was universally applauded as the (albeit ugly-as-sin) crown jewel of chemistry. It would be a monumental effort to overturn it. I also explained that I wasn't a great one to endorse anything because my work in astronomy is pretty far off the beaten path (sacred calendars are just not mainstream astronomy) and so I didn't think I could help her. I know she was disappointed, but she continued her campaign to get anyone to listen. I don't think she had much success.
Now Helen has passed on to where she might be able to chat personally with Einstein and Bohr. But of course, up there they probably get to know what's really going on, and won't need to argue much.
Recently I had a dream, one of those realistic kind that one is tempted to take seriously. In it I was a professor at a University, and into my office came a woman who looked a whole lot like Helen. In my dream I was very concerned about just who she was and what her credentials were. She kept trying to show me some charts, but when I learned she was "just" a homemaker, I wasn't interested and turned her away.
When I awoke, I felt it had been a big mistake to turn that woman away. It appeared to refer to Helen because that is just what happened and I now am retired and am not associated with a university. I thought that if it was a warning it was a couple of decades too late.
So what was my surprise when shortly afterward I met another woman named Kathryn Paulsen who had been given the baton to keep alive Helen's discoveries and to continue trying to raise some interest in the whole concept (see orderintheatom.blogspot.com). She is mentioned in Helen's book as someone who really was encouraging. So, with that dream in mind, I decided not to botch my opportunity again, but instead to take another look at it. As I did, I remembered that I have a web page on the Periodic Table which is used all over the world and is more popular than any of my articles on sacred calendars. So here I am reviewing Helen's work and putting her chart up on my website.
When Helen took chemistry, she was struck as probably most of us were, at how awkward the usual periodic table is. For those not familiar with that table of all the elements, it would be good to look at the shape of the table, perhaps even on this web site (see "Periodic Table Memory Pegs"). She believed that Nature is not ugly, but is organized (that's what keeps scientists in business), and later it became almost an obsession with her to create an aesthetically pleasing table. (Others have also wanted to make a circular table, but if you do an internet search, you'll find they all look like the regular table with duct tape sticking the two ends together! They couldn't get "away from the boxes".)
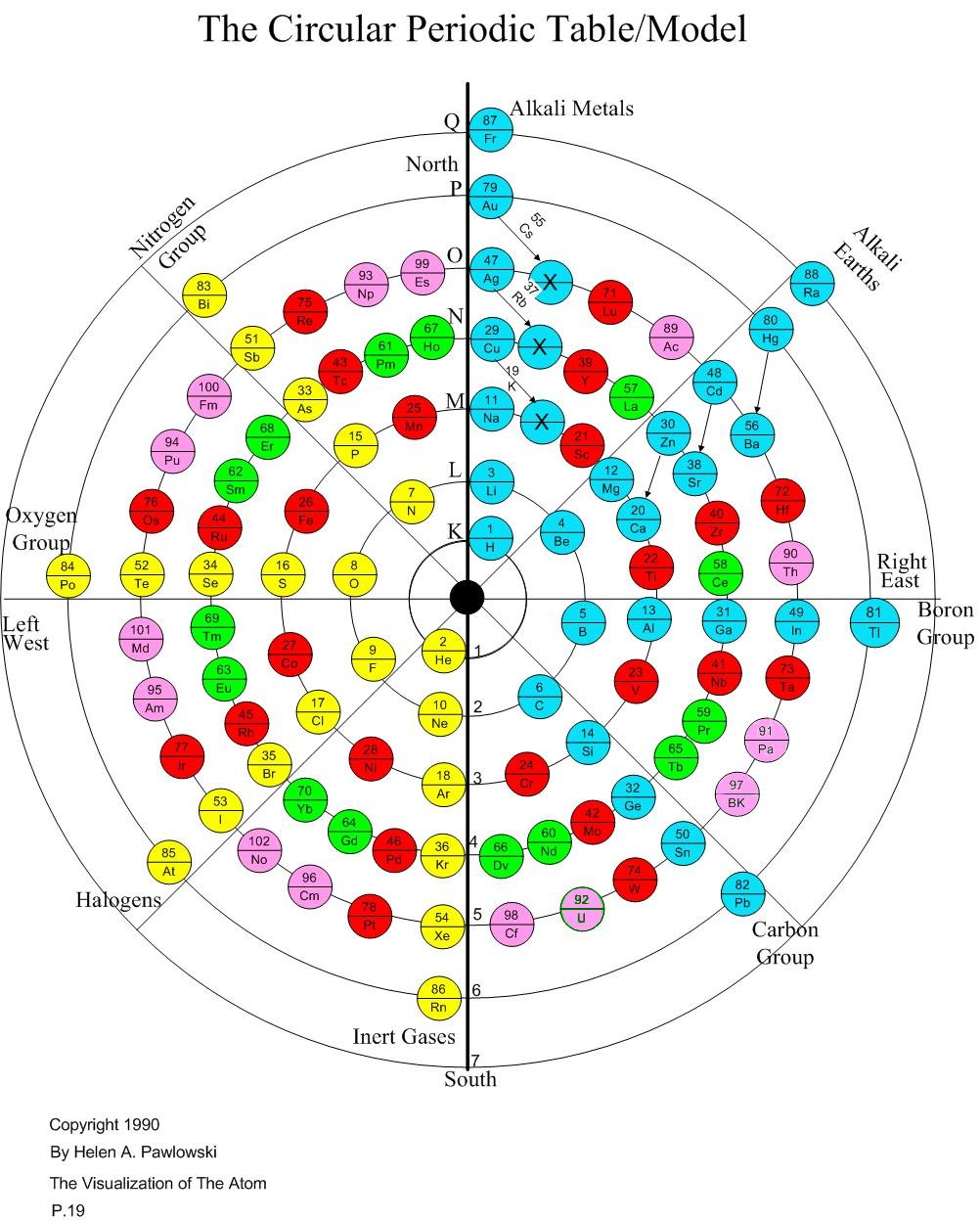
With aesthetics as a starting point, and knowing that there was something magic about the number "8", which is still not explained at all by science and which is what gave rise to the periodic table to begin with, she began to fill in a circular table that has eight main "rays". As she did so, she came across the idea of putting in the elements in the place where the "distinguishing electron" (as she calls it) drops into its place in its "shell" (Bohr model) or principal quantum number (quantum mechanics).
That is pretty much the heart of the matter (no pun intended!). She found she could not just place the atoms in a continuous circular fashion, but that there was apparently some sort of "polarity line" down the center. So after going in a clockwise rotation for elements 3-6, she needed to switch to counter-clockwise for 7-10. Take a look at her chart, and look the the placement of the elements with those numbers on the second shell to understand that concept.
 |
One would have to admit that Helen achieved what was her original goal of producing a beautiful and very symmetrical chart. Most people feel that Nature should be organized and elegant and this table fulfills that purpose well.
What led to the first Periodic Table was the discovery that there was a periodicity that centered around the number 8. That has never been understood, even with all the work in quantum mechanics. This chart emphasizes that when any shell after the first has its first 8 slots filled and is electrically neutral, then that the element becomes a "noble gas" which doesn't readily react with any other element. In Helen's chart, these gases are aligned in the vertical row at the lowest point of the shells. They are Helium, Neon, Argon, Krypton, Xenon, and Radon. These elements do not all have the outer shell filled, but rather they all have the outer 8 slots filled. Of course, in the case of Neon, 8 slots complete the outer (second) shell.
One of Helen's successes is her discovery of "sliders". There are six elements that are placed at the end of the transitionals in a standard Periodic Table. She points out they do not belong there, especially because the valence of copper, silver and gold is one, rather than two, as with the others. Moreover, what she calls the "distinguishing electron" is in the top shell rather than the lower shells as in the other transitionals. So she decided those nine elements could share the spot for "top dog" in the shell with the alkali metals and alkali earths.
Helen's work goes well beyond just making a pretty table. She takes the polarity line very seriously. It is similar to saying that there is no spherically symmetric ground state of hydrogen, but that hydrogen has the filled slot on just one side of the atom. That would leave the other side open, which she suggests explains "hydrogen bonding". That is a strong prediction, which if verified, would greatly strengthen her claim that this chart is actually a model of the atom.
In modern textbooks, alchemy is always presented as the clumsy, inept, early attempt to understand what is now the advanced science of chemistry. In my understanding it is the other way around. There is much evidence that alchemists did indeed transmute lead into gold. Gold was considered the "king of the metals" and indeed it still is considered best by most people. It has absolutely amazing characteristics, being tops in malleability, ductility, and lack of corrosion. By the way, after laughing at the alchemists, the modern texts often have a footnote that says that by pure chance lead is next to gold on the periodic table and that in fact lead has been transmuted into gold in small quantities in modern accelerators!
Helen's chart puts gold at the top, in what might seem like its proper place to an alchemist, rather than buried in the transitional metals. Something about that seems right to me, even though gold is not highly reactive like the alkali metals. At the end of this article, I put my memory icons into Helen's arrangement, and it turns out that three of her Group 1 sliders are what are used for U.S. coins: copper, silver and gold.
Helen's "sliders" were probably the feature that was most strongly rejected by those of academia. They argued that it was only the usual alkali metals of potassium, rubidium and cesium that are highly alkaline like sodium, and that it was nonsense to put copper, silver and gold in those places.
To me the answer to this challenge is to ask what is the purpose of the chart. The purpose of the traditional Periodic Table seems to be to align in vertical columns elements with similar chemical affinities, and similar valences. That works great for the alkaline metals and the halogens and inert gases. But it begins to fall apart for everything else. For example, nitrogen exhibits all 8 possible valences, so if the table were about valences, nitrogen would have to be in all eight main columns!
Helen's chart is not so much about valences, although it also shows valences well for those principal elements. It is more a chart of the atomic structure of the atom. Because that is her purpose, her chart works great to see the order in which the slots are filled for different atoms.
Helen's chart does not show the valences of all the transitional elements, but as mentioned above it is not a primary goal to show all valences. In the case of transitionals, it is pretty easy to memorize that they nearly all have a valence of two. Overall to me Pawlowski's chart is a dramatic improvement over the traditional chart.
Helen definitely succeeded in her primary goal of creating a balanced, aesthetically pleasing chart. The result is a hybrid between a model and a periodic table. It is not perfect at being either, but it is an excellent compromise which both shows the structure of the atomic shells well as well as many of the valences of the principal elements. It represents a major improvement over the traditional table. It certainly merits consideration as a teaching tool to become part of any introductory course in chemistry or atomic structure. In case anyone is interested, I decided to make a chart with her arrangement and my icons.